
Clinical support & resources for COVID-19 management
Access support for products, including cleaning and disinfecting information, video tutorials and more.

Products & services for COVID-19 management
Explore a comprehensive portfolio of product and service solutions for managing COVID-19 patients.
Ventilators & respiratory care
Philips offers a broad portfolio of respiratory solutions that include invasive and noninvasive ventilation ranging from mid- to high-acuity, oxygen therapy, CPAP and BiPAP therapy, nebulizers, and masks. We are actively engaged with our customers throughout the globe to facilitate access to these solutions, knowing they can help clinicians, hospitals and health systems as they navigate this complex and dynamic environment.
(Please note: not all products are available in all geographies. Please check with your Philips representative or call us at 1-800-345-6443 for complete portfolio availability.)
Featured ventilator and respiratory care products
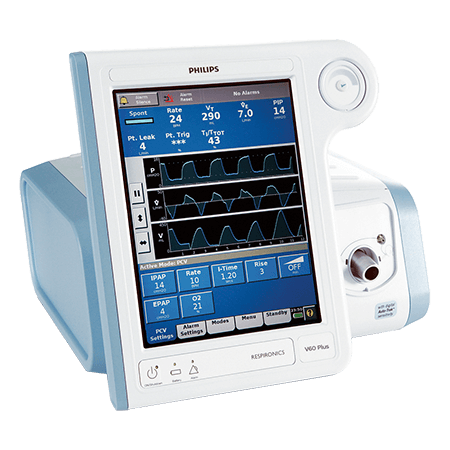
V60 Plus1
The V60 Plus gives care providers flexibility to address a wide variety of respiratory needs efficiently. The device has the capability to provide noninvasive ventilation (NIV), invasive mechanical ventilation (IMV) and high flow therapy (HFT) care.
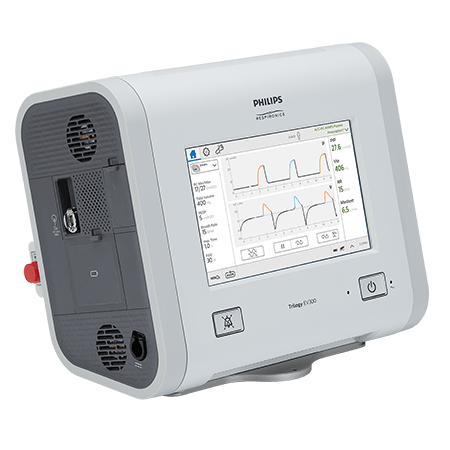
Trilogy EV300
Trilogy EV300 is a versatile ventilator that can support treatment in varying care spaces within the hospital - from the emergency department to the ICU. Additionally, Trilogy EV300 is part of the Trilogy Evo platform of ventilators, the only life-support ventilator platform designed to stay with patients and provide consistent therapy and monitoring as their conditions and care environments change.
Ventilation
Sleep therapy
Disposable masks with non-vented options
Reusable masks
Respiratory drug delivery
Airway clearance
Oxygen
Learn more about our hospital to home care continuum
We're with you

Organized to assist

Actively adapting
Creating connections
References 1 Philips V60 Plus ventilator software upgrade is provided in the US under an FDA emergency use policya, which authorizes its use for the duration of the COVID-19 public health emergency declaration, after which the software upgrade may no longer be used. This device is not FDA cleared or approved. a Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency, issued March 2020

